
Topics
 |
|
Lecture Video
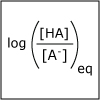
A buffer helps to maintain a constant pH. Our blood has a natural buffering system to ensure that the pH of our blood stays within a narrow window and that we stay health. In this lecture, we consider how to design a buffer. We also discuss how one can predict the pH of a salt solution. At dinner if you put table salt in your water glass, how would the pH of the water change? or would it change?
> Download from iTunes U (MP4 - 113MB)
> Download from Internet Archive (MP4 - 113MB)
Lecture Notes
Clicker Questions
Lecture 22 Clicker Questions (PDF - 1.1MB)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Autoprotolysis and pH | Sections 11.13 and 11.18–11.19 | Sections 10.13 and 10.18–10.19 |
| Mixed Solutions and Buffers | Sections 12.1–12.3 | Sections 11.1–11.3 |
