
Topics
 |
|
Lecture Video
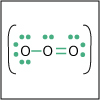
Lewis structures are simplistic views of molecular structure. They are based on the idea that the key to covalent bonding is electron sharing and having each atom achieve a noble gas electron configuration. Lewis structures correctly predict electron configurations around atoms in molecules about 90% of the time. They are not perfect, but writing a Lewis structure is a lot easier than solving the Schrödinger equation, so we recommend that you watch this lecture.
> Download from iTunes U (MP4 - 97MB)
> Download from Internet Archive (MP4 - 97MB)
Lecture Notes
Clicker Questions
Lecture 10 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Lewis Structures | Section 2.5–2.8 | Section 2.5–2.8 |
