
Topics
 |
|
Lecture Video
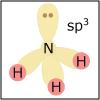
Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. In particular, the concept of hybridization is important for understanding the geometry of organic molecules.
> Download from iTunes U (MP4 - 126MB)
> Download from Internet Archive (MP4 - 126MB)
Lecture Notes
Clicker Questions
Lecture 14 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Valence Bond Theory | Sections 3.4, 3.5, 3.6, and 3.7 | Sections 3.4, 3.5, 3.6, and 3.7 |
