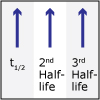
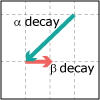
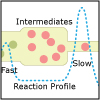
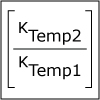
In Unit V, we consider the speed, or rate, of chemical reactions. Thermodynamics tells us if a particular reaction will be spontaneous, but not how fast it will occur. Thus, both thermodynamics and kinetics are necessary for understanding chemical reactions. By the end of this unit, viewers should be able to analyze kinetics data to determine the order of the reaction with respect to any reagent, write simple reaction mechanisms, and determine if a mechanism is consistent with experimental data. They should be familiar with the steady-state approximation, rate-determining steps, and activation energy barriers. Viewers should be able to describe the properties of a catalyst and be familiar with terms associated with enzyme catalysis.
Looking for something specific in this course? The Resource Index compiles links to most course resources in a single page.