
Topics
 |
|
Lecture Video
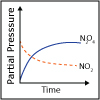
Reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. In this lecture, we discuss the nature of chemical equilibrium and of the chemical equilibrium constant. We start to consider how external factors can “push” the equilibrium in one direction or the other. Physicist and Chemist Nozomi Ando provides an example of why chemical equilibrium is important in living organisms.
> Download from iTunes U (MP4 - 107MB)
> Download from Internet Archive (MP4 - 107MB)
Lecture Notes
Clicker Questions
Lecture 18 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Chemical Equilibrium | Sections 10.1–10.5, and 10.9 | Sections 9.1–9.4 |
Related Behind the Scenes at MIT Videos
Understanding Chemotherapeutic Drug Targets
Nozomi Ando conducts research on a protein that is essential for DNA synthesis, repair, and replication. She explains how this protein is in equilibrium between an active and an inactive form, and how discovering strategies to lock the protein in the inactive conformation could lead to treatments for cancer and/or could be used in the creation of new antibiotics.
Nozomi Ando, a self-described artist and comic book lover, describes how her training to become a scientist was akin to that of a ninja. Like a ninja apprenticeship, expert scientists have worked with her at every stage of her career to show her the way of the scientist.
