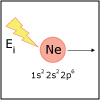
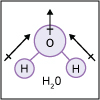
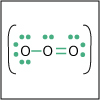
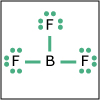
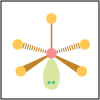
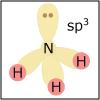
With the knowledge of atomic electronic configurations and ionization energies from Unit I, Unit II focuses on how (and why) atoms come together to form bonds and how (and why) certain molecular structures are formed as a result of bonding interactions. The unit starts with the periodic table where all the elements are introduced. Viewers are asked to consider why elements on one side of the table react by losing electrons whereas elements on the other side have the tendency to gain electrons, leading to the description of properties known as electron affinity and electronegativity. Viewers will be introduced to ionic, covalent, and polar covalent bonds, and to a theory that can predict bond strength (molecular orbital theory). Viewers will also learn about molecular structure, and theories that predict which arrangement of atoms and electrons is likely to yield the most stable molecule (Lewis structures), and which geometry is most likely to be observed (VSEPR and valence bond theory/hybridization). By the end of unit II, viewers should be able to identify periodic table trends, draw Lewis structures, and use molecular orbital theory to explain why it takes extremely high temperatures to break the nitrogen-nitrogen bond of dinitrogen. They should be able to use VSEPR theory to explain why the greenhouse gas carbon dioxide is linear, identify which organic molecule is likely to have a tetrahedral center, and explain to their parents or kids which vitamins are polar and thus safe to take in higher doses.
Looking for something specific in this course? The Resource Index compiles links to most course resources in a single page.