
Topics
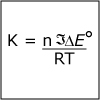 |
|
Lecture Video
Viewers are introduced to agents of oxidation and agents of reduction. Are oxidizing agents really that bad for you? Hear from Professor John Essigmann about the double-edged sword that is oxidation-reduction. Oxidizing agents can protect us from disease but can also damage our genetic material. Friend or foe, you decide.
> Download from iTunes U (MP4 - 98MB)
> Download from Internet Archive (MP4 - 98MB)
Lecture Notes
Clicker Questions
Lecture 26 Clicker Questions (PDF)
Textbook Reading
| TOPICS | 5th EDITION | 4th EDITION |
|---|---|---|
| Galvanic and Electrolytic Cells | Sections 13.6–13.12 | Sections 12.6–12.12 |
Related Behind the Scenes at MIT Videos
Linking Oxidation To DNA Damage
John Essigmann describes how oxidation reactions in our bodies are both essential for life and responsible for cell damage that can potentially lead to cancer. John’s research focuses on studying how cells respond to toxins that cause oxidative damage to DNA.
John Essigmann’s Personal Story
John Essigmann describes how an early industry experience doing real science pushed him to pursue a career as a professor and professional scientist. He also realizes that the Scientific Method is a framework that can be applied to better understanding questions in the real world.
