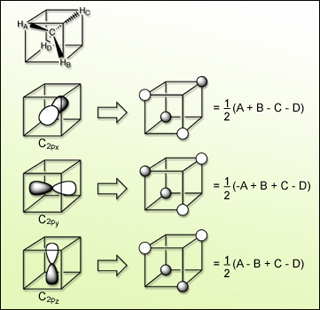
Linear combinations of H 1s atomic orbitals that match nodal properties of C 2p atomic orbitals for tetrahedral methane. (Figure by MIT OpenCourseWare.)
Instructor(s)
Prof. Christopher Cummins
Prof. Sylvia Ceyer
MIT Course Number
5.112
As Taught In
Fall 2005
Level
Undergraduate
Translated Versions
Course Description
Course Features
Course Description
5.112 is an introductory chemistry course for students with an unusually strong background in chemistry. Knowledge of calculus equivalent to MIT course 18.01 is recommended. Emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis. The course also covers applications of basic principles to problems in metal coordination chemistry, organic chemistry, and biological chemistry.


